If you’ve ever wondered, do dentists use new tools for each patient?, and what really happens behind the scenes to keep everything safe, you’re not alone. Many patients assume they know the answer, but the truth is more detailed than most expect. In this article, you’ll uncover the exact protocols dentists follow, and the one safety step that might surprise you most.
Review sterilization steps with a dentist in Queens Blvd.
TL;DR:
Dentists do not reuse tools without safeguards. Reusable instruments go through a strict, multi-step process, cleaning, inspection, packaging, sterilization, monitoring, and proper storage, before each patient, while disposable items are used once and discarded. Patient safety depends on standardized protocols, trained staff, continuous monitoring, and compliance with regulatory and manufacturer guidelines to prevent cross-contamination.
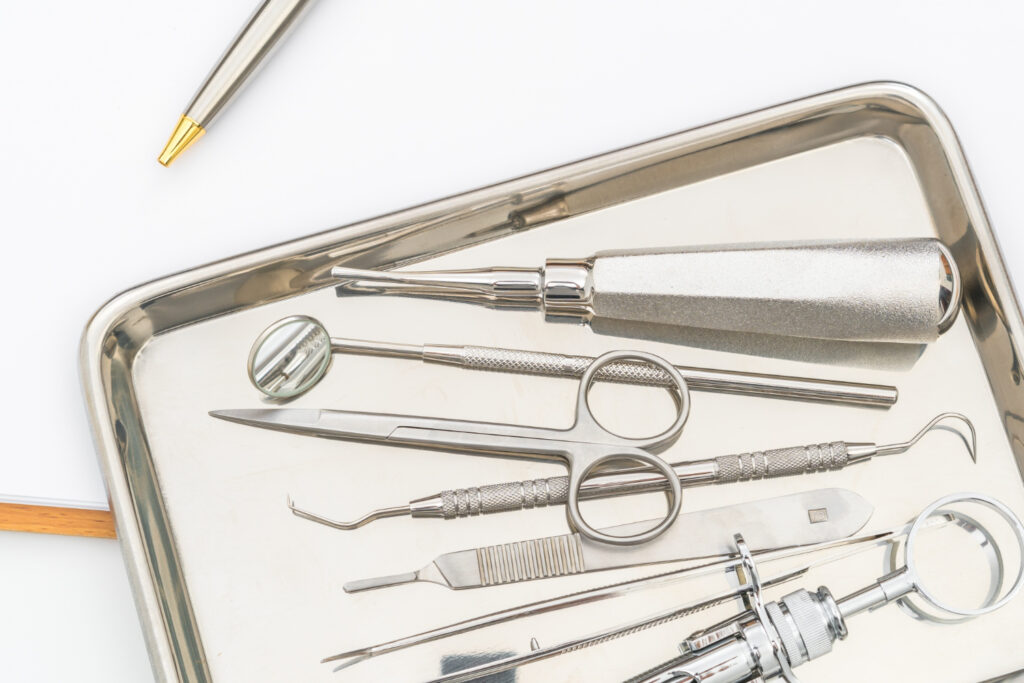
How Do Dentists Sterilize Their Tools Between Patients?
Dentists use a structured, multi-step sterilization process to ensure every instrument is safe before it touches a new patient. This process begins the moment instruments leave the mouth and continues through cleaning, inspection, sterilization, and proper storage—all supported by strict monitoring and documentation.
- Point-of-use cleaning and transport
Immediately after use, instruments are wiped to remove visible debris and placed in a secure container or tray. This prevents contamination of surfaces and staff while also keeping blood and fluids from drying, which makes the next cleaning steps more effective. - Cleaning
Cleaning removes soil and microorganisms so sterilization can work properly. Practices often use ultrasonic cleaners or instrument washer-disinfectors for consistent results. Heavily soiled or complex instruments may be brushed or flushed before mechanical cleaning. This step significantly reduces bioburden and is required before sterilization. - Inspection and packaging
After cleaning, instruments are examined for damage and reassembled if needed. They are then packaged in sterilization pouches or wraps that allow the sterilant to penetrate while keeping instruments sterile afterward. Chemical indicators, which change color during sterilization conditions, are placed inside or on the package for verification. - Sterilization
Steam sterilization (autoclaving) is the most widely used and reliable method, applying controlled time, temperature, and pressure to inactivate microorganisms, including spores. Other validated methods, such as dry heat or chemical vapor, may be used for appropriate instruments. All steps must follow manufacturers’ reprocessing instructions. - Monitoring and documentation
Effectiveness is checked at three levels: mechanical monitoring (cycle time, temperature, pressure), chemical indicators, and biological indicators that test whether spores survive. Routine monitoring, maintenance logs, and proper recordkeeping are essential for safe, compliant infection control. - Storage and handling
Sterilized packs are stored in a clean, dry area and handled carefully to maintain sterility. They remain sealed until opened at the point of care for the next patient.
Disposable vs. Reusable Dental Instruments
Dental practices rely on both disposable and reusable instruments, each serving different purposes in maintaining safety, efficiency, and cost-effectiveness. Comprehending the differences helps clarify why some items are used only once while others are sterilized and reused. The comparison below highlights how each option impacts workflow, infection control, environmental footprint, and long-term value.
| Category | Disposable Instruments & Barriers | Reusable Instruments |
| Definition | Single-use items discarded after one patient. | Instruments designed for repeated cleaning and sterilization. |
| Examples | Needles, plastic mirrors, suction tips, control barriers. | Scalers, probes, forceps, many handpieces, restorative tools. |
| Pros | Eliminates reprocessing errors; reduces turnaround time; lowers cross-contamination risk; useful for heat-sensitive or complex items. | More economical long-term; lower waste; high-quality options often only available as reusables. |
| Cons | Higher ongoing supply costs; increased environmental waste; performance varies by design. | Requires reliable reprocessing infrastructure; improper sterilization poses safety risks; ongoing monitoring and maintenance needed. |
| Best Use | When single-use design improves safety or practicality, or when reprocessing isn’t feasible. | When strong, validated cleaning and sterilization systems are in place and manufacturer instructions can be followed consistently. |
Ensuring Patient Safety Through Proper Hygiene
Patient safety in dental care relies not just on sterilizing instruments correctly, but on maintaining strong system-level practices that ensure consistency, accountability, and rapid response when needed. Effective hygiene depends on coordinated protocols, trained staff, reliable monitoring, and transparent communication, all working together to minimize risk.
- Standardized protocols and checklists
Written procedures for cleaning, sterilization, monitoring, storage, and handpiece reprocessing help eliminate variability and reduce errors. Checklists and logs create a clear trail of accountability and ensure each step is completed correctly. - Staff training and competency
All team members involved in instrument handling or reprocessing must be properly trained and regularly updated. Training covers correct cleaning methods, loading sterilizers, interpreting chemical indicators, when to run biological tests, and steps to take when failures occur. Competency assessments and refresher sessions support consistent, safe performance. - PPE and environmental controls
Proper use of gloves, masks, and eye protection reduces cross-contamination risks during instrument handling. Environmental measures support sterility efforts and provide an additional layer of protection. - Monitoring and rapid response to failures
Mechanical, chemical, and biological monitoring systems detect sterilization issues early. Clear protocols guide what to do when a failure occurs: remove affected loads, reprocess instruments, investigate possible causes, and document corrective actions. While sterilizers generally perform reliably, prompt response to failures is essential to preventing patient exposure. - Patient communication and transparency
If a sterilization breach affects a patient, regulatory frameworks often require timely notification and clear documentation of follow-up steps. Strong internal reporting and incident-response policies not only protect patient health but also help maintain trust and meet public-health responsibilities.
Regulatory Guidelines for Dental Tool Sterilization
Regulatory expectations for dental sterilization come from multiple sources, including public-health authorities, occupational-safety agencies, professional associations, and device manufacturers.
Government health agencies provide the core infection-control guidance, outlining how instruments must be cleaned, which validated sterilization cycles are acceptable, and what monitoring and documentation are required. These rules form the baseline standards that dental practices must follow to ensure patient safety.
Professional dental organizations supplement these rules with practical tools, such as checklists and device-specific recommendations, helping offices apply regulations during day-to-day operations. Their guidance often clarifies the proper handling of sensitive devices and reinforces the importance of following evidence-based protocols aligned with national standards.
Manufacturers’ instructions and regulatory clearances also play a central role. Each device comes with validated reprocessing instructions, and practices must follow them exactly, particularly when a tool cannot tolerate heat sterilization.
Many regions further require audits, recordkeeping, and training documentation to verify compliance. Together, these layers create a comprehensive system that protects both patients and staff.
Key Takeaways
- Dentists follow a multi-step sterilization process to ensure every reusable instrument is safe for the next patient. Mechanical, chemical, and biological checks verify that sterilization conditions were met.
- Disposable and reusable tools serve different roles in infection control. Disposables reduce cross-contamination risk and turnaround time, while reusables are more economical long-term but require strict, validated reprocessing systems to remain safe.
- Patient safety depends on system-level hygiene practices, such as standardized protocols, thorough staff training, proper PPE use, and environmental surface controls. These elements minimize human error and strengthen overall infection prevention.
- Monitoring and rapid response protocols protect patients when sterilization failures occur. Defined steps ensure issues are contained and corrected quickly.
- Regulatory guidelines shape sterilization standards, with public-health agencies, professional organizations, and manufacturers providing requirements for cleaning, validated cycles, monitoring, and recordkeeping. Compliance with these overlapping rules maintains safety and accountability in dental settings.
FAQs:
Do dentists use different tools for each patient?
Yes. Dentists either use new disposable tools or fully sterilized reusable instruments for every patient to ensure safety and prevent cross-contamination.
Do dentists reuse instruments?
They reuse only instruments designed for multiple use, and these undergo a strict cleaning, inspection, sterilization, and monitoring process before being used again.
Are dentist tools single use?
Some are. Items like needles, certain plastic mirrors, suction tips, and protective barriers are single-use and discarded after one patient.
Do dentists clean tools between patients?
Absolutely. Reusable instruments are thoroughly cleaned, packaged, sterilized, monitored, and stored before being used on the next patient.
Sources
Vatanparast, B., Buitrago, J. M., & Siqueira, M. F. (2024). Exploring sterilizer performance through external biological indicator testing: a retrospective study. BMC oral health, 24(1), 1361. https://doi.org/10.1186/s12903-024-05152-2
Patiño-Marín, N., García, L. D. V., López, E. C. A., Medina-Solís, C. E., Zumarán, A. M., Rider, R. M., … & Salas Sr, M. (2025). Sterilization and Disinfection: Ensuring Infection Control in Dental Practices. Cureus, 17(2). 10.7759/cureus.79041
You May Also Like:
Dentist for Cavity Under Filling in Forest Hill, Queens NY